The Quick Takeaway for Busy Procurement Pros
For the busy healthcare procurement leader, the bottom line is this: switching to safety-engineered syringes isn’t just a regulatory checkbox—it’s a strategic imperative. While the upfront cost may be higher, this investment delivers a powerful return on investment (ROI) by drastically reducing the immense direct and indirect costs of needlestick injuries, ensuring OSHA compliance, and fundamentally strengthening your organization’s culture of safety and patient protection. It’s a shift from short-term cost-cutting to long-term value creation.
Hey, Let’s Chat. Does This Scene Sound Familiar?
Your desk is a fortress of spreadsheets and contracts, your days a high-wire act balancing fiscal prudence with clinical necessity. You master the logistics, you negotiate the deals, and you keep the hospital running. But what if one of the most ubiquitous items in your catalog—the humble syringe—is a Trojan horse? What if this seemingly benign tool carries a latent, devastating risk not just to the budget, but to the very people you’re tasked with equipping? A single, preventable prick can ignite a catastrophic chain reaction of financial and human costs, completely eclipsing the line-item savings you fought so hard to secure. This isn’t just about a minor incident; it represents a silent fiscal hemorrhage and a profound breach of our duty of care. The crucial question is no longer whether conventional syringes are good enough, but whether they have become fundamentally indefensible for today’s healthcare leader. Let’s look at what’s really at stake.
The Hidden Danger in Plain Sight: The Real Story of Needlestick Injuries
More Than Just a “Prick”: What a Needlestick Injury Actually Entails
We often talk about needlestick injuries, or NSIs, in clinical, detached terms. But for a healthcare worker, it’s a moment of terror. Beyond the initial puncture, it’s the agonizing wait that follows—days and weeks of uncertainty. This accidental exposure to bloodborne pathogens is a serious occupational hazard. It brings the immediate threat of disease transmission, including life-altering infections like HIV, Hepatitis B (HBV), and Hepatitis C (HCV). The psychological stress and emotional distress for healthcare workers are immense, impacting their well-being, job satisfaction, and ability to care for patients. This isn’t a minor workplace incident; it’s a profound, preventable occupational hazard that echoes through a person’s life.
According to the World Health Organization (WHO), over two million healthcare workers experience percutaneous injuries with contaminated sharps annually, leading to a substantial risk of infection.
Let’s Talk Numbers: The Staggering Stats You Can’t Ignore
The frequency of sharps-related injuries is alarming. The CDC estimates that around 385,000 needlestick injuries occur each year in U.S. hospital settings alone. Nurses are disproportionately affected, but any personnel handling sharps—from doctors to lab technicians to housekeeping staff—are at risk. These aren’t just numbers on a report; each one represents a potential for cross-contamination, a personal crisis for an employee, and a failure in our system of healthcare worker protection. Effective injury prevention strategies are not optional; they are a core responsibility.
The True Cost of “Sticking” with Conventional Syringes

The Direct Hit: Unpacking the Financial Fallout of an Injury
The financial implications of a single needlestick injury are staggering. A cost-benefit analysis of safety devices quickly reveals the economic burden of inaction. Direct costs include immediate medical care, extensive laboratory testing for the exposed worker and the source patient, and the high cost of post-exposure prophylaxis (PEP) for HIV and HBV. These medical treatment costs injury can quickly escalate, and when you factor in potential worker’s compensation claims and the direct medical cost per needlestick injury, the financial hit to your institution can run into thousands, or even tens of thousands, of dollars.
The Ripple Effect: Hidden Costs That Damage Your Organization
Beyond the immediate medical bills, the indirect costs of NSI can be even more damaging. These hidden costs include productivity loss due to absenteeism and time spent on incident reporting and follow-up. It can lead to increased staff turnover, as employees feel their workplace safety is compromised, incurring further recruitment and training costs. Furthermore, the risk of litigation costs is ever-present. An institution seen as failing to provide adequate protection faces not just financial penalties but also significant damage to its reputation, eroding public trust and patient confidence. This is a financial burden that no healthcare financial management team can afford to ignore.
Meet the Game-Changer: An Introduction to Safety-Engineered Syringes
This is where advanced safety syringe technology comes in. Safety-engineered devices (SEDs) are specifically designed with built-in safety mechanisms to prevent needlestick injuries. These are not just minor upgrades; they represent a fundamental shift in injection safety.
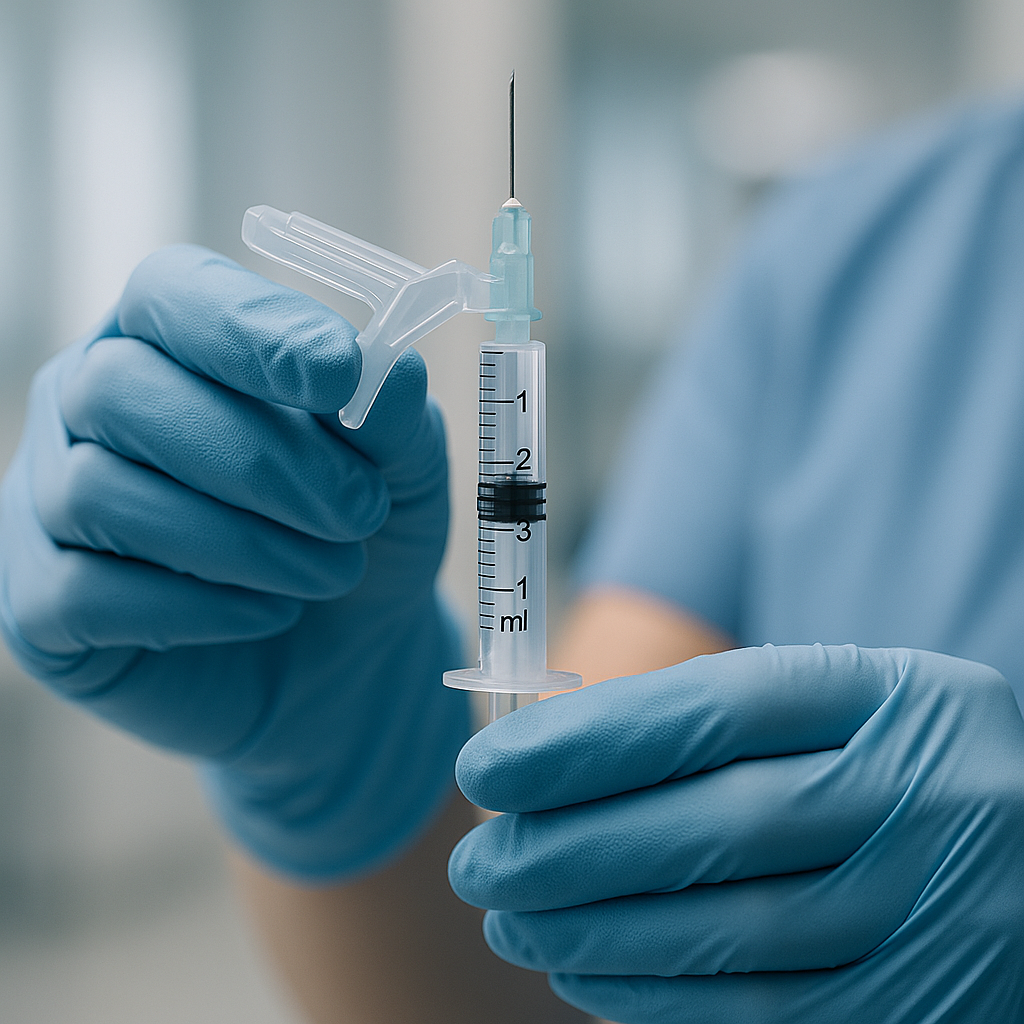
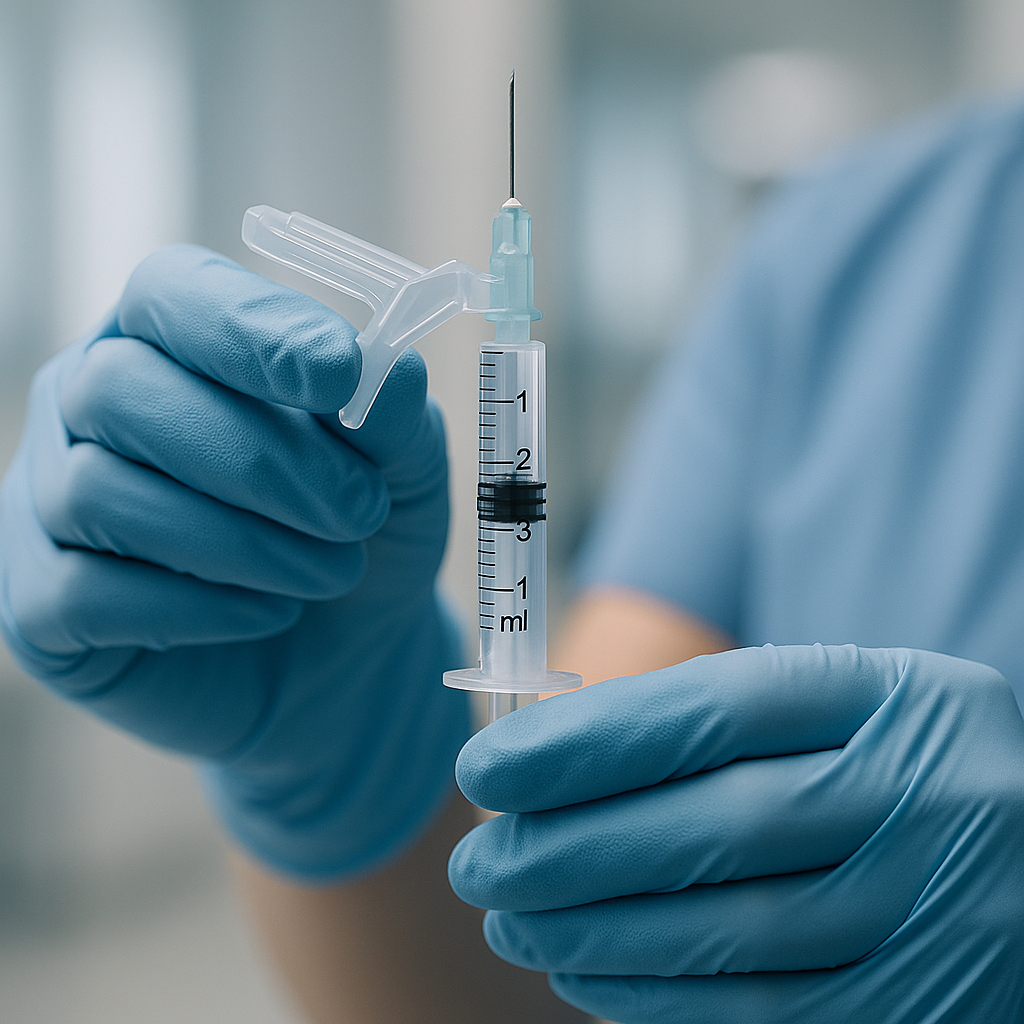
So, How Do They Actually Work? A Look at the Main Types
Safety-engineered sharps come in several innovative designs, primarily categorized by their safety features:
- Passive Safety Features: These mechanisms are automatic, requiring no extra steps from the user. For instance, retractable needle syringes automatically withdraw the needle back into the barrel after the injection is complete. This intuitive design offers the highest level of protection as it eliminates exposure immediately and requires no change in aseptic technique.
- Active Safety Features: These require the user to manually activate the safety mechanism. Examples include sliding sheaths or protective shields that the user slides over the needle after use, or hinged safety caps that are snapped into place. While effective, they rely on user action, which can sometimes be forgotten in a fast-paced environment.
Other safety innovations include single-use syringes with a breaking plunger to prevent reuse, further enhancing patient safety and reducing cross-contamination risks.
Are They Really More Effective? What the Evidence Shows
The data is overwhelmingly clear. Numerous studies and reports from organizations like the CDC and NIOSH have demonstrated that the implementation of safety-engineered devices is directly linked to a dramatic reduction in NSI risk. Facilities that have adopted a comprehensive prevention program, including the use of safety syringes and sharps with engineered sharp injury protection (SESIP), report sharps injury reduction rates of up to 80% or more. This is compelling evidence that investing in safer medical devices translates directly into a safer environment for both staff and patients.
The ROI of Safety: Why This Is a Smart Financial Move, Not Just an Expense
Shifting from a Cost Center to a Value Investment
Thinking about safety syringes solely in terms of their higher upfront cost is a classic procurement pitfall. The real financial picture emerges when you conduct a total cost of ownership analysis. The premium paid for safety devices is an investment that yields substantial returns. The long-term financial benefits, driven by the avoidance of direct and indirect injury costs, create a compelling return on investment (ROI) for safety syringes. This is the essence of value-based procurement: making purchasing decisions that deliver the best overall outcome and net savings, not just the lowest price per unit.
The economic evaluation of safety devices consistently shows a favorable budget impact when the full lifecycle costs of needlestick injuries are considered.
Keeping the Regulators Happy: The Compliance Advantage
In the United States, the Needlestick Safety and Prevention Act mandated OSHA to strengthen its Bloodborne Pathogens Standard (29 CFR 1910.1030). This regulation requires employers to identify, evaluate, and implement safer medical devices, including safety-engineered sharps. Similar regulations, like the EU Directive on sharps injuries, exist globally. Adhering to these regulatory standards isn’t just about avoiding hefty fines for non-compliance; it’s about upholding a legal and ethical duty of care. Shifting to safety syringes is a clear and defensible step toward meeting regulatory compliance for devices and fostering a robust safety culture in healthcare.
“But What About…?” Answering Your Top Concerns
Objection #1: “The Upfront Cost is Too High.”
This is the most common objection, and it’s understandable. Budgets are tight. However, framing it as “pay now or pay much, much more later” is more accurate. A detailed cost-benefit analysis of safety syringes will show that the cost of a single NSI—including medical expenses, lost productivity, and potential litigation—can easily outweigh the entire incremental cost of switching a department to safety devices for a year. It’s a classic case of preventative spending that yields significant long-term savings.
Objection #2: “Our Staff Isn’t Trained for New Devices.”
Change can be challenging. However, modern safety syringes are designed with human factors engineering and user-centered design principles in mind. Many passive safety devices are incredibly intuitive and require little to no change in technique. A crucial part of your procurement strategy should involve supplier collaboration. A good supplier will not just sell you a product; they will partner with you, providing comprehensive staff training, in-service education, and support materials to ensure a smooth and successful device implementation.
Making the Switch: Your Playbook for a Seamless Transition

Your Checklist: What to Look for in a Safety Syringe and a Supplier
When undertaking device selection, your value analysis committee should consider several key factors:
- Reliability & Effectiveness: Is the safety mechanism robust and dependable?
- Ease of Use: Is the device intuitive? Does it require minimal change in technique?
- Regulatory Clearance: Is the device FDA-cleared, CE-marked, or compliant with relevant ISO standards?
- Clinical End-User Involvement: Involve nurses and other frontline workers in the product evaluation process. Their feedback is invaluable.
- Supplier Support: Does the vendor offer robust training, support, and a stable supply chain?
Rolling It Out: A Simple 3-Step Plan for Implementation
A phased rollout can make the transition feel manageable and ensure success.
- Pilot Program: Select a single unit or department to pilot the new devices. Gather data and feedback on usability and effectiveness.
- Staff Training & Feedback: Use insights from the pilot to refine your training program. Hold hands-on sessions and address all staff concerns before a wider rollout.
- Full Rollout & Monitoring: Implement the new devices facility-wide. Continue to monitor for any issues and track sharps injury rates to demonstrate the program’s success to all financial stakeholders.
Our Final Thought: It’s More Than a Purchase, It’s a Statement
Ultimately, the decision to standardize safety syringes goes beyond contracts and line items. It’s a powerful statement about your organization’s values. It says that you prioritize patient safety first. It declares that you are committed to protecting the healthcare workers who dedicate their lives to caring for others. In today’s healthcare landscape, shifting your procurement strategy to embrace safety-engineered devices is not just a smart business decision—it’s the right thing to do.